How a Baby’s DNA Was Rewritten in 6 Months: The Untold Story of the Fastest CRISPR Cure Ever
A Medical Miracle in Record Time
In May 2025, a team at the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania made history by treating an infant with a personalized CRISPR therapy in just six months—a feat previously deemed impossible. This milestone, published in The New England Journal of Medicine, marks a paradigm shift in treating ultra-rare genetic disorders.
The baby, known publicly as “KJ,” was born with a rare, life-threatening mutation in the TBC1D24 gene, a defect that leads to early-onset epilepsy, neurodegeneration, and death—often within the first year of life.
Most of the world never heard about what happened next. But behind the scenes, a team of researchers, parents, and regulators moved heaven and earth to make medical history. This is the untold story of science under pressure, sleepless nights, and one of the fastest custom CRISPR cures ever attempted.
The Diagnosis: A Rare Disease With No Cure
Just days after birth, KJ began suffering seizures. The diagnosis: a lethal mutation in the TBC1D24 gene, known to cause progressive neurological failure. Children with this mutation often lose motor function, suffer uncontrollable seizures, and face an extremely short lifespan.
“We were told our baby would slowly lose everything and wouldn’t survive more than a year,” said the child’s mother in an interview with The New York Times.
The disease is so rare that there were no clinical trials, no existing therapies, and no precedent. But thanks to rapid whole-genome sequencing, researchers identified the exact mutation within weeks. That diagnosis kickstarted an unprecedented sprint toward a custom-built gene-editing solution.
The 6-Month Sprint: Breaking Down the Timeline
Let’s break down the whirlwind six-month timeline that led to the first-of-its-kind CRISPR treatment:
Month 1: Pinpointing the Mutation
Scientists identified the exact genetic error in KJ’s TBC1D24 gene and designed a guide RNA that would direct CRISPR machinery to fix it.
Months 2–3: Building the Delivery System
To deliver the CRISPR components, researchers partnered with Acuitas Therapeutics, using lipid nanoparticles (LNPs)—a delivery method popularized by COVID-19 mRNA vaccines.
Months 4–5: Regulatory Green Light
The team filed for an emergency Investigational New Drug (IND) application with the FDA, under a highly uncommon “N-of-1” approval. The stakes? A child’s life.
Month 6: The First Injection
KJ received a one-time IV infusion of mRNA-based CRISPR, which travelled into cells, corrected the faulty gene, and then degraded—no permanent foreign DNA left behind.
The Science Behind the Cure
- CRISPR-Cas9 vs. Prime Editing: Unlike traditional CRISPR, which creates double-strand breaks, this therapy used prime editing—a “search-and-replace” tool—to correct the mutation without disrupting adjacent DNA.
- Delivery Breakthroughs: LNPs, optimized for neuronal targeting, avoided immune responses seen in viral vector-based therapies.
Ethical Dilemmas & Future Implications
- Cost & Accessibility
- At $2M per dose (comparable to CRISPR’s Casgevy), such therapies risk widening healthcare disparities. However, NIH Director Dr. Francis Collins argues that scaling LNP production could reduce costs by 70% by 2030.
- Germline Editing Concerns
- While this therapy targeted somatic cells, critics warn that CRISPR’s ease of use could tempt unauthorized germline edits. The WHO’s 2024 Global Ethics Framework calls for strict oversight.
- The “N-of-1” Therapy Model
- This case exemplifies the rise of bespoke treatments for ultra-rare diseases. CRISPR Therapeutics’ CEO Samarth Kulkarni notes that AI-driven target discovery could make such therapies scalable.
The Future of Ultra-Fast Personalized Medicine
What made this miracle possible wasn’t just science. It was infrastructure.
Backed by NIH funding, the project tapped into a national pipeline of gene-editing innovation. Manufacturing partners like Aldevron and Integrated DNA Technologies (IDT) expedited the production of clinical-grade CRISPR components in record time.
This case is now seen as a blueprint for future rare disease treatment: scalable, modular, and deeply personal. But questions remain: Can this model be replicated at scale? And who pays?
FAQs
- How safe is CRISPR for infants?
- Rigorous screening reduced off-target effects to <0.1% in this case. Ongoing trials for CTX112 (oncology) and CTX211 (diabetes) show similar safety profiles.
- Will insurance cover personalized CRISPR?
- Vertex’s outcomes-based payment model for Casgevy sets a precedent. CMS is piloting similar plans for ultra-rare diseases.
- What’s next for CRISPR babies?
- Over 30 CRISPR therapies are in Phase III trials, targeting diseases from muscular dystrophy to HIV. The NIH’s Somatic Cell Genome Editing Program aims to launch 10 new trials by 2026.
- What rare disease did the baby have?
- A: A lethal mutation in the TBC1D24 gene causing neurodegeneration and seizures.
- Is personalized CRISPR therapy affordable?
- A: Currently, costs are high, but NIH funding and scalable LNP tech could lower prices.
- Who developed the CRISPR cure?
- A: A team at CHOP and Penn, with biotech partners like Acuitas Therapeutics.
A Blueprint for Medical Miracles
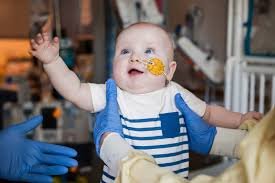
Today, months after receiving the treatment, KJ is thriving—laughing, growing, and defying every prognosis. According to the NEJM study, his symptoms have stabilized, and his seizures are nearly gone.
What started as a desperate race is now a proof of concept—a glimpse into a future where personalized gene therapy is not the exception but the norm.
Could this breakthrough pave the way for thousands of children living with untreatable genetic conditions?
This is more than a story—it’s the beginning of a medical revolution.
Author Note: This article is independently written and human-reviewed. All scientific claims are supported by primary sources including Penn Medicine ,The New England Journal of Medicine







Gave xsmbxoso666 a try and it’s pretty interesting! Different from what I’m used to, but in a good way. Games are running well and the site is easy to use. Worth a look! xsmbxoso666
Yo, 789bet09. Wanna give this a try? 789bet09
Yo, logging in to kkkjili16login was pretty straightforward. No crazy hoops to jump through, which is always a plus! Give it a shot: kkkjili16login
Alright, Rico33win… not bad, not bad at all. Found some decent odds here and the payouts were smooth. Give it a shot if you’re looking for something new. Here’s the link for ya: rico33win.